Mechanism of Ammonia Loss in Swine Effluent
Sang Ryong Lee1, Wayne P. Robarge1* and John T. Walker2
Swine animal feeding operations (AFOs) are sources of various gases [ammonia (NH3), hydrogen sulfide (H2S), carbon dioxide (CO2), volatile organic compounds (VOCs)], and fine particulate matter. A key for understanding NH3 emission in swine waste effluent has been suggested by the equilibrium equation:
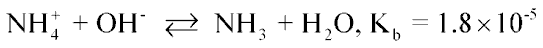
Gaseous emissions from simple aqueous systems are typically controlled by temperature, pH, wind speed, total dissolved concentration of the chemical species of interest (e.g. NH4+NH3 = TAN), and the Henry’s law coefficient. Using a simple flow-through Teflon-lined chamber (0.3m × 0.2m × 0.15m), we have compared emissions of NH3 from swine barn and lagoon effluent (finisher facilities, eastern North Carolina) to pure solutions of (NH4)2SO4 under conditions of identical pH, temperature and TAN. Wind speed in the chamber is < 0.05 m sec-1. All treatments exhibit the expected response in emissions with changes in temperature and pH. However, NH3 emissions from the barn liquid are 5 times those from pure solutions of (NH4)2SO4 (figure 1).
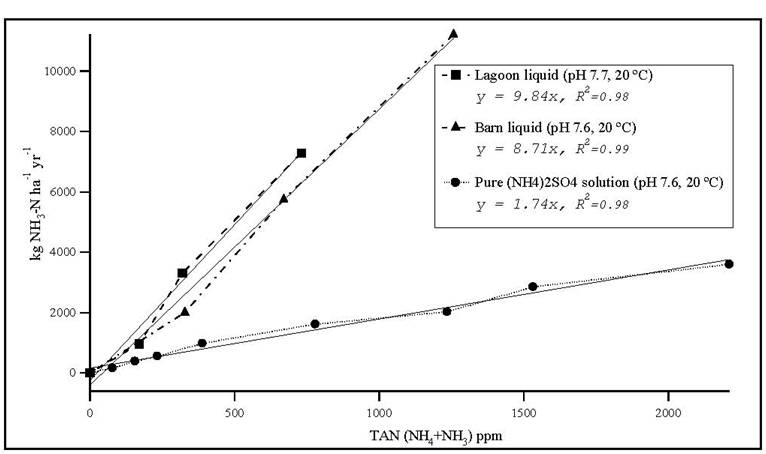
Figure 1. Ammonia flux from swine lagoon and barn liquid compared to an ammonium sulfate solution.
The large difference in emissions between solutions of equivalent TAN suggests the possibility of a synergistic mechanism that is enhancing NH3 emissions. Little is known about the interactions of other gases, i.e. NH3-VOCs which contribute to further loss of NH3 in swine AFOs. This presentation will propose an alternative NH3 volatilization mechanism in swine waste effluent. Concurrent measurements as part of the National Air Emissions Monitoring Study (NAEMS) document the emissions of CO2, H2S and VOCs (primarily acetic, propionic and butyric acids) at levels that are comparable to observed NH3 emissions. Controlled experiments are underway to investigate further the potential for enhanced emissions of NH3 due to the concurrent release of other volatile species. If successful, this work will be extended to determine the potential for other synergistic reactions that influence gaseous emissions across the air-liquid interface of swine manure effluent.
* Wayne P. Robarge: ; 919-515-1454
1 North Carolina State University, Department of Soil Science, Raleigh, NC 27695-7619
2 U.S. Environmental Protection Agency, NRMRL, Research Triangle Park, NC 27711